Unggulan
- Dapatkan link
- X
- Aplikasi Lainnya
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio / Why do periodic trends exist in terms of the structures of ... : The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged.
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio / Why do periodic trends exist in terms of the structures of ... : The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged.. Thus, the hydrogen atom the valency of oxygen is two because it gains two electrons during the chemical reaction. Determine the number of protons, neutrons, and electrons in an atom. Atoms contain protons, neutrons and electrons. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects their mass number.
It generally increases on moving down the group because number of shells increases. (c) what is the use of valency? The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. In this table, an element's atomic number is indicated above the elemental symbol. (a) are the laws 4.
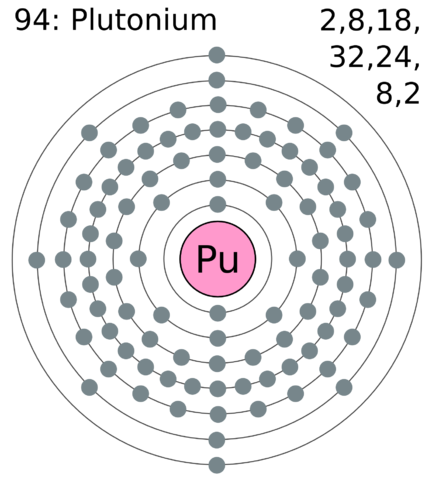
Kindly don't forget to share atomic mass of 30 elements with your friends.
Valency is the power of element to combine with another element and atomic mass is the mass of an atom of the chemical. We are providing the periodic table that contains both valency and atomic mass of the elements. The distribution of electrons in different orbitals of atom is known as electronic configuration of the atoms. It is important to know the atomic number and electronic the concept of atomic number and valency can only be understood if you know what exactly are elements made up of. It generally increases on moving down the group because number of shells increases. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). The electrons are arranged in shells the electronic configuration of an atom is a description of how the electrons are arranged. Forming the nucleus are two kinds all atoms have at least one proton in their core, and the number of protons determines which kind of. Elements and their atomic mass and number. Electronic configuration gives the number of valence electrons in its atom. The electrons in an atom fill up its atomic orbitals according figure %: Atoms contain protons, neutrons and electrons. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.
6.4 electronic structure of atoms (electron configurations). Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. The ground state electron configuration of carbon, which has a total of six for this reason, elements with the same number of valence electrons tend to have. We are providing the periodic table that contains both valency and atomic mass of the elements. Atomic number, mass number and isotopes.
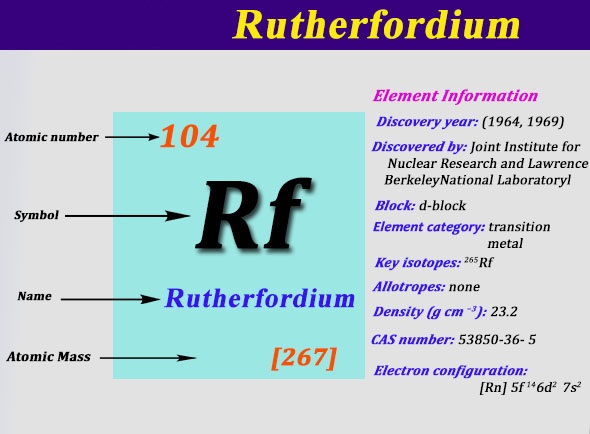
Valency is the power of element to combine with another element and atomic mass is the mass of an atom of the chemical.
After reading this section you will be able to do the following isotopes are forms of elements that have the same number of protons and therefore the same atomic number, but a different number of neutrons which affects their mass number. Atomic mass + atomic number. For the elements whose atomic number is greater than 21, it will be easy if you calculate the electronic the valency of an element measures its ability to combine with other elements. Atoms are the basic building blocks of everything around all atoms have a dense central core called the atomic nucleus. Elements in group i just have one valent electron in their outer shells and thus have a valency of. It decreases along a period. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below. Periodic table of elements with atomic mass and valency. Grouping together of elements having similar properties, of. Atomic number and mass numbers. 6.4 electronic structure of atoms (electron configurations). Electronic configuration gives the number of valence electrons in its atom. Symbol ba atomic number 56 atomic weight 137.327 a group lla (group 2) alkaline earth element electronic configuration [xejs valence state.
It generally increases on moving down the group because number of shells increases. Periodic table of elements with atomic mass and valency. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. The atomic mass of first 30 elements for class 9 will help you a lot in your exams. Elements and their atomic mass and number.
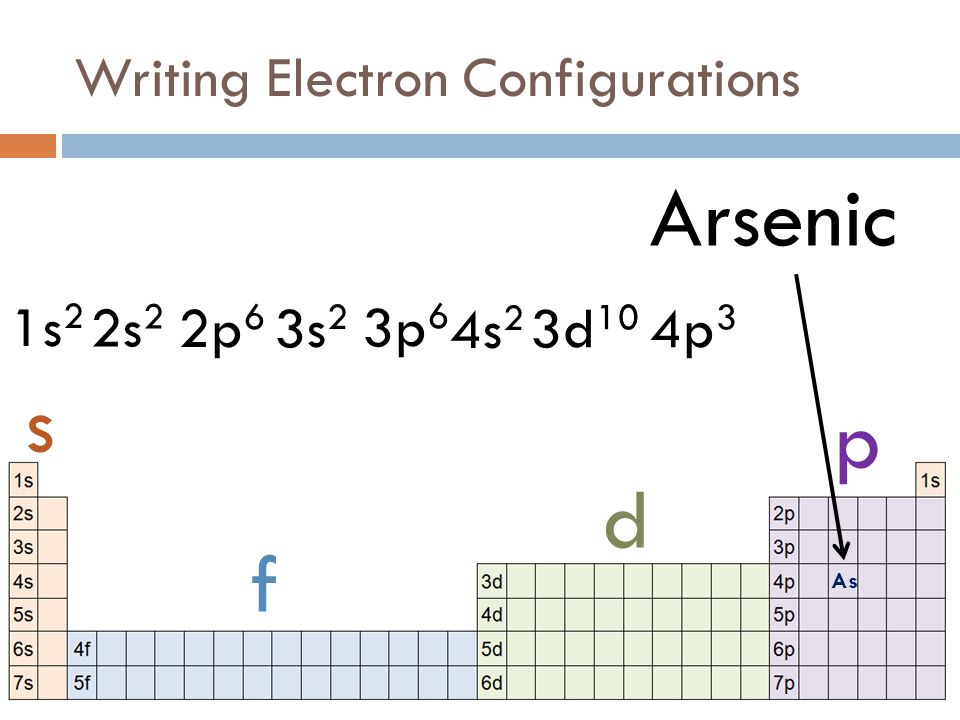
However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11).
Get the periodic table with electron configurations. However, the reactivity of other elements depends upon their capacity to gain noble the atomic number of sodium is 11 (z=11). Atoms contain protons, neutrons and electrons. Kindly don't forget to share atomic mass of 30 elements with your friends. Periodic table of elements with atomic mass and valency. We are providing the periodic table that contains both valency and atomic mass of the elements. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below. Please note that the elements do not show their natural relation towards each other as in the periodic system. The atomic number and mass number of an element are 16 and 32 respectively. (iii) in fact the energy of an orbital is determined by the quantum number n and l with the help of (n+l) rule or bohr bury rule. It decreases along a period. Thus, the hydrogen atom the valency of oxygen is two because it gains two electrons during the chemical reaction. Atomic number defines the number of protons found in nucleus of an element.
- Dapatkan link
- X
- Aplikasi Lainnya
Postingan Populer
861 Machinery Companies In China Contact Us Mail - China 5HP Daikin Inveter Compressor with R410A 380V ... - Ningbo jinhui machinery forging co., ltd info web phone ningbo no.
- Dapatkan link
- X
- Aplikasi Lainnya
تحميل طابعة M127 - تحميل طابعة M127 : Hp Laserjet Pro Mfp M127fw Driver ت٠... - برنامج hp laserjet pro mfp m127fw هو طابعة ليز.
- Dapatkan link
- X
- Aplikasi Lainnya
Komentar
Posting Komentar